The prompt partner in biotec research.
eDNA partners with organizations dedicated to innovation in human tissue research. Using the partners’ scientific and clinical expertise, we have developed global collaborations and have implemented protocols for obtaining, handling, processing and analysis of human specimens worldwide. The primary focus of clinical trials are oncology, including solid tumours and hematologic malignancies; but we also conduct projects in neurology, inflammation, metabolic and cardiovascular diseases, maternal health and infectious diseases.
Our Services
High Quality Fixed Tissue Specimens for Research
eDNA BioBank provides access to thousands of ready-to-go fully consented or consent exempt archival FFPE samples. These specimens have been prepared in strict compliance with our standard operation procedure (SOP) requirements and according to our ISO13485 certified Quality Management System for medical devices.
Our standard procedure implies a small piece of human tissue to be prepared by a certified medical pathologist, submerged in 10% neutral-buffered formalin for 18 – 24 hours, and embedded in IHC-grade paraffin. The target FFPE specimen will generally allow preparation of 100+ sections and have at least 5×5 mm2 size, but it can vary significantly depending on the nature of the disease and tissue type. The fixation agent and the embedding media can be customized upon request in prospective collections.
Fresh Frozen Tissues for RNA, DNA and Protein Work
eDNA Biobank and Network provides access to fully pre-screened and consented human samples in the FF (Fresh-Frozen) tissue format. These specimens have been prepared in strict compliance with our standard operation procedure (SOP) requirements and according to our ISO13485 certified Quality Management System for medical devices.
Our standard procedure implies a a small piece of tissue prepared by a certified medical pathologist is snap-frozen in liquid nitrogen within 30 min after the surgical excision. A standard sample weighs 100 – 500 mg on average and is supplied in a standard 2 ml cryovial. An embedding media (like RNA preserving additives) can be added upon request in prospective collections; a mirroring FFPE block to represent the frozen tissue may be available.
Frozen Bone Marrow Aspirates
o Solid Oncology: Tumour and Adjacent Normal Tissues, blood and blood derivatives
o Hematologic Oncology (AML, CML, ALL, AML, MM, MDS): Bone marrow, blood and blood derivatives
o Inflammation and Autoimmune Diseases: Skin, Synovial tissue, blood derivatives, others
o Neurological Diseases: Tissue and blood derivatives for diseases of CNS and peripheric neurons
o Normal Controls: Various matching tissue samples from otherwise healthy patients in other indications; blood derivatives from healthy controls and bone marrow aspirates from non-malignancy conditions.
Fresh specimens
eDNA provides surgical tissues to be delivered in best non-frozen cold ischemic conditions to most places in Europe and Middle East in 24 hours of removal. Accompanying whole blood or blood derivatives may also be available in a non-frozen form. Our standard operation procedure (SOP) requirements are strictly applied for best viability, but custom procedures can be followed in collection, processing or transportation.
Fresh tissues are always delivered to address using a special courier to address, as all customs procedure is completed in delivered duty paid (DDP) form, if not requested otherwise.
Specimen Sets
Most fresh frozen tissue samples can be ordered with their matching FFPE block. Some samples also have their frozen preoperative blood derivatives.
Most FFPE blocks can be ordered with their adjacent normal tissue (ANT) specimens. Any prospective collection can be customised for collection of multiple specimens and data from same source. Matching controls can be acquired from healthy or otherwise healthy individuals of same sex and ±2-5 years age.
Frozen Whole Blood and Blood Derivates
Whole blood from eDNA Biorepository is supplied as a 2 – 10 ml frozen blood sample in a standard K2-EDTA tube. Serum/Plasma is obtained from whole blood using standard blood fraction separation procedures provided by collection tube manufacturers. The anti-coagulation agent can be adjusted to customer request in prospective collections.
The specimens are generally supplied as snap-frozen aliquots of 0,5 or 1 ml in standard 2 ml cryovials. Subcellular fractions from blood (including viable frozen cells) can be prepared upon request. Also, other tubes and packaging are available upon request.
Viable Cells
eDNA also provides specimens of peripheral blood and bone marrow mononuclear cells (PBMC and BMMC) or dissociated solid tumour cells that have been prepared in strict compliance with our standard operation procedure (SOP) requirements to achieve best viability. Custom procedures can also be applied.
Our standard procedure for mononuclear cells implies processing and freezing of specimens in 2 hours of collection, maintaining a 95-98% viability proven by the standard Tryphan Blue counting method. Viable mononuclear cells are available as 5 Million cells per ml in a suspension of a cell banking medium. Viable tumour cells are available in various forms and rates depending on the nature of the tissue and disease. Procedures or media can be adjusted to project needs in prospective collections.
Medical Data
Medical Data always complements the biospecimens in eDNA BioBank. Various demographic data, medical records of patient including disease background, details of the condition, treatment history, family history may be available or can be acquired in prospective collection based projects. A complete pathology report is always available for tissue specimens and other reports like diagnostic imaging studies may be provided.
Medical data is always handled pursuant with the The Regulation (EU) N°910/2014 on electronic identification and trust services for electronic transactions in the internal market (eIDAS Regulation), ISO/IEC 27001:2013 Information Technology Security Techniques, IMS Requirements and ISO22301:2019 Security and resilience, as well as Turkish Personal Data Protection Law no. 6698.
Logistics and Customs Processing
Fresh tissues are always delivered to address in 24 hours, using a special courier to address, as all customs procedure is completed in delivered duty paid (DDP) form, if not requested otherwise.
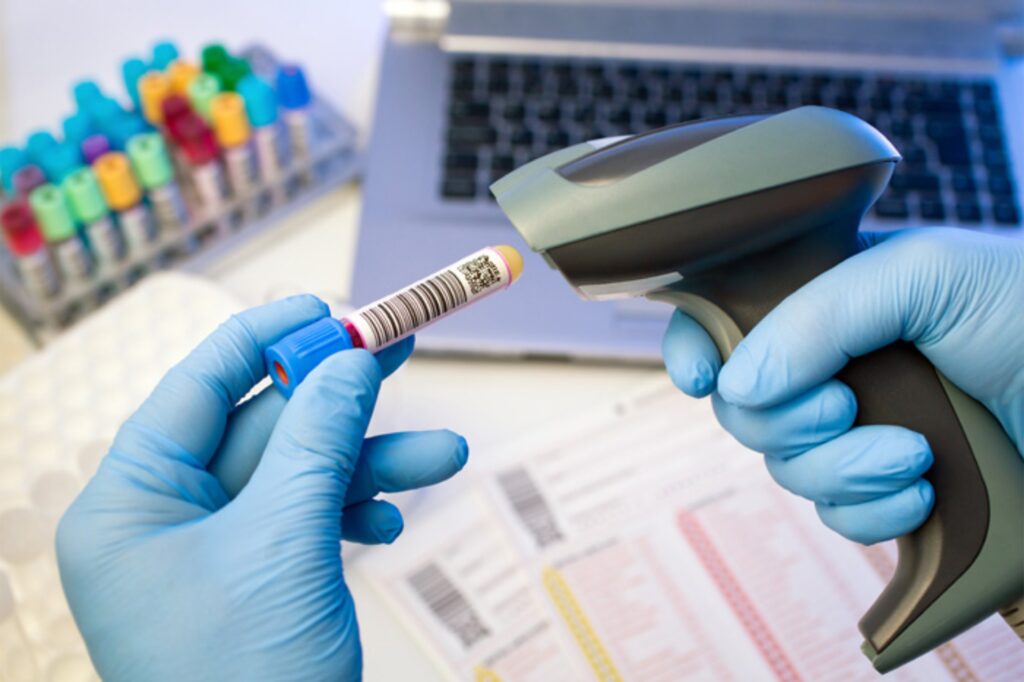
Complete Traceability
eDNA BioBank provides a game changing approach for identifying the banked biospecimens. All history of the specimen, including origin of the specimen, the collection and processing date and times, applied SOPs for collection, storage and transportation, ethical approval documents, customs documents; as well as the complete available data about patient, diagnosis and follow-up can be reached by simply reading the unique barcode on an individual cryovial, collection tube or tissue cassette.
Other Human Biospecimens
eDNA Biobank works closely with principal investigators and project managers on developing custom protocols, conducting feasibility studies and managing biospecimen acquisition projects. Specimen acquisition protocols for the collection of clinically defined biospecimens also include the following indications:
Metabolic Diseases: Diabetes Mellitus (Type I and Type II) collection of diabetes blood, serum, plasma samples from patients with defined clinical criteria.
Gynaecologic Conditions: Collection of endometrium and ectopic endometrium biopsies from patients with confirmed endometriosis and from a non-endometriosis control group, collection of cervical or vaginal swaps or vaginal bleeding samples.
Cardiovascular Conditions: Atherosclerosis, Myocardial Infarction, Cardiac Failure, Coronary Artery Disease matched serum samples, as well as surgical tissue specimens as available.
Maternal and Fetal Health: Early non-invasive diagnosis studies and preterm birth or genetically inherited conditions registries. Clinical outcome data are also available for longitudinal collections.
Custom Projects
eDNA BioBank works closely with our customers to create a unique custom study design for every custom biobanking protocol allowing to achieve specified research objectives in the evidence-based and a cost-effective manner. We prioritise ethical, medical, and technical considerations including patient enrolment criteria, sample format and preparation, data collection and database entry methods.
In addition, our protocol development experts will ensure that your study will be scientifically rigorous and meet the Ethical Committee’s requirements in the applicable jurisdictions. We have worked with the following protocols in the past:
o Drug RnD – from Discovery to Preclinical models
o Diagnostics – from Biomarker discovery to Companion Diagnostics
o Medical devices – from concept to clinical testing
We are committed to apply your study design considering all your needs for Study Objectives (SOW), project specifications (Inclusion/Exclusion criteria; HBS specifications; laboratory HBS processing protocols), clinical data (de-identified general medical history and laboratory tests data; project specific CRF; DB); logistics, deliverables, and timelines; budget and payment schedule.
About Us
eDNA partners with organizations dedicated to innovation in human tissue research. Using the partners’ scientific and clinical expertise, we have developed global collaborations and have implemented protocols for obtaining, handling, processing and analysis of human specimens worldwide. The primary focus of clinical trials are oncology, including solid tumours and hematologic malignancies; but we also conduct projects in neurology, inflammation, metabolic and cardiovascular diseases, maternal health and infectious diseases.

Contact Us
Please fill in the form to send us an e-mail.

eDNA Teknoloji Araştırma ve Geliştirme San Tic Ltd.

Tekeli m. ITOB OSB 10032 s.. 2/A201 Bilimpark, Menderes 35865 İzmir, TR